The Lewis structure of carbon dioxide is given below. The hybridization of the carbon atom in carbon dioxide is ________. 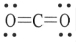
A) sp3
B) sp2
C) sp
D) sp2d
E) sp2d2
Correct Answer:
Verified
Q46: A typical triple bond _.
A)consists of one
Q59: The N-N bond in HNNH consists of
Q61: Based on molecular orbital theory, the bond
Q62: Based on molecular orbital theory, the bond
Q64: An antibonding π orbital contains a maximum
Q67: Based on molecular orbital theory,the bond order
Q67: The hybridization of carbon in the H-C
Q70: In comparing the same two atoms bonded
Q74: According to MO theory,overlap of two s
Q77: A molecular orbital can accommodate a maximum
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents