There is/are ________ π bond(s) in the molecule below. 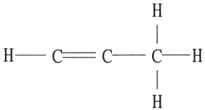
A) 7
B) 6
C) 2
D) 1
E) 0
Correct Answer:
Verified
Q108: The molecular geometry of the H3O+ ion
Q113: In order to produce sp3 hybrid orbitals,_
Q114: The Lewis structure of carbon monoxide is
Q117: There is/are _ σ bond(s)in the molecule
Q118: The hybridization of orbitals on the central
Q120: In order to produce sp2 hybrid orbitals,_
Q121: The bond angle marked a in the
Q121: The electron-domain geometry and molecular geometry of
Q130: Using the VSEPR model,the electron-domain geometry of
Q136: The electron-domain geometry and molecular geometry of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents