Using the van der Waals equation, the pressure in a 22.4 L vessel containing 1.00 mol of neon gas at 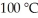 is ________ atm. (a = 0.211 L2-atm/mol2, b = 0.0171 L/mol)
is ________ atm. (a = 0.211 L2-atm/mol2, b = 0.0171 L/mol)
A) 0.730
B) 1.00
C) 1.21
D) 1.37
E) 0.367
Correct Answer:
Verified
Q87: The volume of HCl gas required to
Q97: The volume of fluorine gas required to
Q98: The volume of hydrogen gas at 45.0
Q99: A gas mixture of Ne and Ar
Q100: A sample of He gas (3.0 L)at
Q102: CO (5.00 g)and CO2 (5.00 g)were placed
Q107: CO (5.00 g)and CO2 (5.00 g)were placed
Q115: Sodium hydride reacts with excess water to
Q117: SO2 (5.00 g)and CO2 (5.00 g)were placed
Q118: SO2 (5.00 g)and CO2 (5.00 g)were placed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents