Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
A) 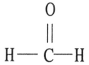
B) 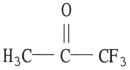
C) 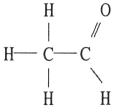
D) 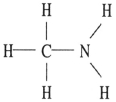
E) 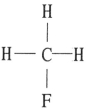
Correct Answer:
Verified
Q3: _ are particularly polarizable.
A)Small nonpolar molecules
B)Small polar
Q4: The strongest interparticle attractions exist between particles
Q6: In liquids,the attractive intermolecular forces are _.
A)very
Q13: When NaCl dissolves in water, aqueous Na+
Q19: A gas is _ and assumes _
Q19: Of the following substances, only _ has
Q20: In which of the following molecules is
Q21: What types of intermolecular forces exist between
Q22: Which of the following molecules has hydrogen
Q31: Based on the following information,which compound has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents