The reaction CH3-N≡C → CH3-C≡N
Is a first-order reaction. At 230.3 °C, k = 6.29 × 10-4s-1. If 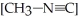 is 1.00 × 10-3 initially,
is 1.00 × 10-3 initially, 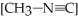 is ________ after 1.000 × 103 s.
is ________ after 1.000 × 103 s.
A) 5.33 × 10-4
B) 2.34 × 10-4
C) 1.88 × 10-3
D) 4.27 × 10-3
E) 1.00 × 10-6
Correct Answer:
Verified
Q3: Of the following,all are valid units for
Q4: What is the order of the reaction
Q6: The rate constant of a first-order process
Q7: The reaction A → B is first
Q8: One difference between first- and second-order reactions
Q13: Which one of the following graphs shows
Q15: The rate law of a reaction is
Q15: The following reaction is second order in
Q17: The reaction 2NO2 → 2NO + O2
Follows
Q17: The data in the table below were
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents