At Elevated Temperatures, Methylisonitrile (CH3NC)isomerizes to Acetonitrile (CH3CN): CH3NC (G)
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results. 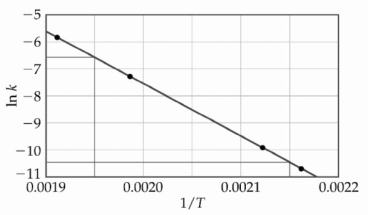 The energy of activation of this reaction is ________ kJ/mol.
The energy of activation of this reaction is ________ kJ/mol.
A) 160
B) 1.6 × 105
C) 4.4 × 10-7
D) 4.4 × 10-4
E) 1.9 × 104
Correct Answer:
Verified
Q21: In general,as activation energy increases,reaction rate _.
A)goes
Q24: Of the following,_ will lower the activation
Q26: The rate of a reaction depends on
Q31: The primary source of the specificity of
Q31: At elevated temperatures, methylisonitrile (CH3NC)isomerizes to acetonitrile
Q32: In the energy profile of a reaction,the
Q34: The mechanism for formation of the product
Q36: The rate law of the overall reaction
Q37: The enzyme nitrogenase converts _ into _.
A)ammonia,
Q40: A possible mechanism for the overall reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents