The value of Keq for the equilibrium H2 (g) + I2 (g)  2HI (g)
2HI (g)
Is 794 at 25 °C. What is the value of Keq for the equilibrium below? 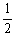 H2 (g) +
H2 (g) + 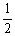 I2 (g)
I2 (g)  HI (g)
HI (g)
A) 397
B) 0.035
C) 28
D) 1588
E) 0.0013
Correct Answer:
Verified
Q23: The equilibrium constant for the gas phase
Q23: The effect of a catalyst on an
Q24: Of the following equilibria, only _ will
Q25: Consider the following reaction at equilibrium: 2NH3
Q26: The equilibrium constant for the gas phase
Q28: Of the following equilibria, only _ will
Q29: How is the reaction quotient used to
Q29: The expression for Kp for the reaction
Q30: Consider the following reaction at equilibrium: 2CO2
Q38: Which of the following statements is true?
A)Q
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents