The value of Keq for the following reaction is 0.50: A (g) + 2B (g)  C (g) + 4D (g)
C (g) + 4D (g)
The value of Keq at the same temperature for the reaction below is ________. 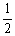 A (g) + B (g)
A (g) + B (g) 
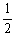 C (g) + 2D (g)
C (g) + 2D (g)
A) 7.1 × 10-1
B) 2.5 × 10-1
C) 0.25
D) 1.0
E) 0.50
Correct Answer:
Verified
Q67: At 1000.0 K, the equilibrium constant for
Q68: The value of Keq for the following
Q69: At elevated temperatures, molecular hydrogen and molecular
Q70: The Keq for the equilibrium below is
Q71: The expression of Keq for the following
Q73: For the endothermic reaction CaCO3 (s)
Q74: The reaction below is exothermic: 2SO2 (g)
Q75: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q76: The value of Keq for the following
Q77: At 900.0 K, the equilibrium constant (Kp)for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents