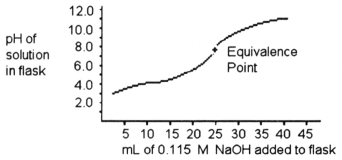
-A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The concentration of the monoprotic acid is about ________ mol/L.
A) 25.0
B) 0.0600
C) 0.240
D) 0.120
E) 0.100
Correct Answer:
Verified
Q24: Which compound listed below has the greatest
Q25: Which one of the following is not
Q34: Human blood is _.
A)neutral
B)very basic
C)slightly acidic
D)very acidic
E)slightly
Q35: For which salt should the aqueous solubility
Q38: A 25.0 mL sample of 0.723 M
Q40: The Ka of benzoic acid is 6.30
Q41: Calculate the percent ionization of formic acid
Q41: The pH of a solution prepared by
Q43: Calculate the maximum concentration (in M)of silver
Q44: The concentration of iodide ions in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents