The first law of thermodynamics can be given as ________.
A) ΔE = q + w
B) Δ 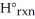 =
= 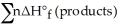 -
- 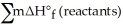
C) for any spontaneous process, the entropy of the universe increases
D) the entropy of a pure crystalline substance at absolute zero is zero
E) ΔS = qrev/T at constant temperature
Correct Answer:
Verified
Q1: The entropy change accompanying any process is
Q6: For an isothermal process, ΔS = _.
A)q
B)qrev/T
C)qrev
D)Tqrev
E)q
Q6: ΔS is positive for the reaction _.
A)2NO
Q7: ΔS is positive for the reaction _.
A)CaO
Q11: The thermodynamic quantity that expresses the extent
Q12: Which one of the following processes produces
Q13: A reaction that is spontaneous as written
Q14: Consider a pure crystalline solid that is
Q18: The entropy of the universe is _.
A)constant
B)continually
Q20: Of the following,only _ is not a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents