Consider the reaction: FeO (s) + Fe (s) + O2 (g) → Fe2O3 (s)
Given the following table of thermodynamic data, 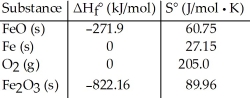 determine the temperature (in °C) above which the reaction is nonspontaneous.
determine the temperature (in °C) above which the reaction is nonspontaneous.
A) This reaction is spontaneous at all temperatures.
B) 618.1
C) 756.3
D) 2438
E) 1235
Correct Answer:
Verified
Q25: For an isothermal process,the entropy change of
Q30: With thermodynamics,one cannot determine _.
A)the speed of
Q34: ΔS is negative for the reaction _.
A)2SO2
Q35: Given the following table of thermodynamic data,
Q37: The value of ΔS° for the reaction
Q37: The equilibrium position corresponds to which letter
Q46: The value of ΔS° for the formation
Q50: The combustion of ethene in the presence
Q52: The value of ΔH° for the decomposition
Q60: The value of ΔS° for the oxidation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents