Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 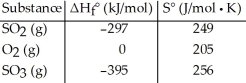
A) 2.40 × 1024
B) 1.06
C) 1.95
D) 3.82 × 1023
E) More data are needed.
Correct Answer:
Verified
Q63: The value of ΔG° at 25 °C
Q64: The value of ΔG° at 25 °C
Q65: The value of ΔH° for the decomposition
Q67: The value of ΔG° at 25 °C
Q69: The value of ΔH° for the formation
Q72: The value of ΔG° at 25 °C
Q74: The value of ΔG° at 25 °C
Q75: The value of ΔG° at 25 °C
Q79: The value of ΔG° at 25 °C
Q80: The value of ΔG° at 373 K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents