Consider the reaction: Ag+ (aq) + Cl- (aq) → AgCl (s)
Given the following table of thermodynamic data at 298 K: 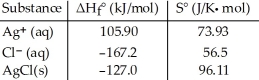 The value of K for the reaction at 25 °C is ________.
The value of K for the reaction at 25 °C is ________.
A) 810
B) 5.3 × 109
C) 1.8 × 104
D) 3.7 × 1010
E) 1.9 × 10-10
Correct Answer:
Verified
Q83: The normal boiling point of water is
Q83: The value of ΔG° at 181.0 °C
Q84: For a reaction to be spontaneous at
Q85: The normal boiling point of ethanol (C2H5OH)is
Q86: Consider the reaction: NH3 (g) + HCl
Q89: The value of ΔG° for a reaction
Q90: Of the following, the entropy of _
Q91: The standard Gibbs free energy of formation
Q92: Of the following, the entropy of gaseous
Q93: Of the following, the entropy of _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents