Table 20.2 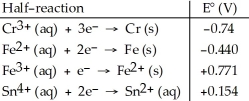
-Which of the following reactions will occur spontaneously as written?
A) Sn4+ (aq) + Fe3+ (aq) → Sn2+ (aq) + Fe2+ (aq)
B) 3Fe (s) + 2Cr3+ (aq) → 2Cr (s) + 3Fe2+ (aq)
C) Sn4+ (aq) + Fe2+ (aq) → Sn2+ (aq) + Fe (s)
D) 3Sn4+ (aq) + 2Cr (s) → 2Cr3+ (aq) + 3Sn2+ (aq)
E) 3Fe2+ (aq) → Fe (s) + 2Fe3+ (aq)
Correct Answer:
Verified
Q5: Which of the halogens in Table 20.1
Q13: Which transformation could take place at the
Q13: Which of the following reactions is a
Q16: What is the coefficient of the dichromate
Q17: What is the coefficient of Fe3+ when
Q17: Consider an electrochemical cell based on the
Q20: Which element is reduced in the reaction
Q21: What is the oxidation number of oxygen
Q23: _ is the oxidizing agent in the
Q32: What is the oxidation number of manganese
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents