The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 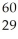 Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
A) 0.5449
B) 1.2374
C) 0.5491
D) 28.7930
E) 1.3066
Correct Answer:
Verified
Q63: In terms of binding energy per nucleon,what
Q69: In balancing the nuclear reaction 
Q71: What percentage of electricity generated in the
Q71: In balancing the nuclear reaction 
Q72: The missing product from this reaction is
Q76: The mass of a proton is 1.00728
Q77: The missing product from this reaction is
Q77: The main scientific difficulty in achieving a
Q79: What exposure level to radiation is fatal
Q79: The missing product from this reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents