Which equation correctly represents what happens when N 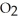 dissolves in water?
dissolves in water?
A) NO2 (g) + H2O (l) → 2H+ (aq) + NO3- (aq)
B) 3NO2 (g) + H2O (l) → 2H+ (aq) + 2NO3- (aq) + NO (g)
C) NO2 (g) + H2O (l) → H2O2 (aq) + NO (g)
D) 2NO2 (g) + H2O (l) → 2H+ (aq) + NO42- (aq) + NO (g)
E) 2NO2 (g) + 2H2O (l) → 2HNO2 (aq) + O2 (g) + H2 (g)
Correct Answer:
Verified
Q42: What are the products of the reaction
Q49: Which of the following would produce the
Q55: Which of the following would produce the
Q57: In the reaction of phosphorus with chlorine
Q60: What is the function of the carbon
Q60: The oxidation number of As in H2AsO3-
Q61: The arrangement of oxygen atoms around a
Q61: Hydrogen can form hydride ions.Elements in group
Q67: The primary commercial use of nitric acid
Q70: A borane is a _.
A)compound containing only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents