In the following titration curve, what does the inflection point represent? 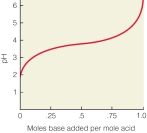
A) pH of solution equals pKa of weak acid
B) concentration of weak acid and conjugate base are equal
C) the pH where the solution would function most effectively as a buffer
D) the weak acid is 50% protonated, 50% deprotonated
E) all of the above
Correct Answer:
Verified
Q8: Which of the following best explains the
Q9: Citric acid is a triprotic acid with
Q10: Given the pKa values for phosphoric acid
Q11: What pH range is generally considered to
Q12: Which of the following represents the breaking
Q14: Which of the following is the conjugate
Q15: Which of the following atoms could interact
Q16: If a buffer is made with the
Q17: Since pKa = -log Ka, which of
Q18: Given the structure of a glucose molecule,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents