What volume (L) of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
A) 50.0
B) 0.44
C) 1.75
D) 0.070
E) 1.75 × 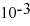
Correct Answer:
Verified
Q41: What volume (mL)of a concentrated solution of
Q46: Which solution contains the largest number of
Q54: What mass (g)of potassium chloride is contained
Q60: What volume (mL)of a concentrated solution of
Q62: _ is an oxidation reaction.
A)Ice melting in
Q64: What mass (g)of barium iodide is contained
Q65: What mass (g)of AgBr is formed when
Q67: A 13.8 mL aliquot of 0.176 M
Q68: What mass (g)of CaF2 is formed when
Q72: What volume (mL)of 7.48 × 10-2 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents