The molarity (M) of an aqueous solution containing 22.5 g of sucrose (C12H22O11) in 35.5 mL of solution is __________.
A) 0.0657
B) 1.85 × 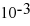
C) 1.85
D) 3.52
E) 0.104
Correct Answer:
Verified
Q84: Which of these metals is the most
Q91: What are the respective concentrations (M)of K+
Q95: Of the following elements, _ is the
Q97: Based on the equations below,which metal is
Q100: Calculate the concentration (M)of sodium ions in
Q108: In a titration of 35.00 mL of
Q111: The molarity of a solution prepared by
Q115: Oxalic acid is a diprotic acid. Calculate
Q121: What is the concentration (M)of sodium ions
Q132: A 31.5 mL aliquot of H2SO4 (aq)of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents