The value of ΔH° for the reaction below is -186 kJ. Calculate the heat (kJ) released from the reaction of 25 g of Cl2. H2 (g) + Cl2 (g) → 2HCl (g)
A) 66
B) 5.3 × 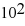
C) 33
D) 47
E) -186
Correct Answer:
Verified
Q30: With reference to enthalpy changes, the term
Q36: Of the substances below, the highest fuel
Q37: Which one of the choices below is
Q46: The kinetic energy of a 7.3 kg
Q50: The ΔE of a system that absorbs
Q53: The value of ΔH° for the reaction
Q57: The change in the internal energy of
Q59: The ΔE of a system that releases
Q66: The value of ΔH° for the reaction
Q67: The value of ΔH° for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents