The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2 H2 (g)
How many kJ of heat are consumed when 15.5 g of CH3OH (l) decomposes as shown in the equation?
A) 0.48
B) 62.0
C) 1.3 × 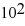
D) 32
E) 8.3
Correct Answer:
Verified
Q53: The value of ΔH° for the reaction
Q56: The value of ΔH° for the reaction
Q57: The change in the internal energy of
Q66: The value of ΔH° for the reaction
Q67: The value of ΔH° for the reaction
Q68: The value of ΔH° for the reaction
Q70: The molar heat capacity of a compound
Q71: The enthalpy change for the following reaction
Q72: The value of ΔH° for the reaction
Q73: The value of ΔH° for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents