Given the data in the table below, ΔH°rxn for the reaction 2 Ag2S (s) + O2 (g) → 2 Ag2O (s) + 2S (s)
Is __________ kJ. 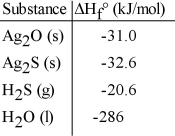
A) -1.6
B) +1.6
C) -3.2
D) +3.2
E) The ΔH°f of S (s) and of O2 (g) are needed for the calculation.
Correct Answer:
Verified
Q103: A meal containing a burger, fries, and
Q113: Given the data in the table below,
Q114: The value of ΔE for a system
Q115: The average fuel value of sugars is
Q116: The kinetic energy of a 12.5-g object
Q118: A slice of apple pie contains 14.0
Q119: The value of ΔH° for the reaction
Q120: The value of ΔH° for the reaction
Q121: The ΔH for the solution process when
Q122: In the presence of excess oxygen, methane
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents