Given the data in the table below, ΔH°rxn for the reaction SO3 (g) + H2O (l) → H2SO4 (l)
Is __________ kJ. 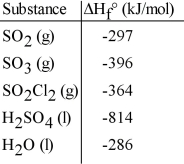
A) -132
B) 1496
C) 704
D) -704
E) -2.16 × 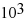
Correct Answer:
Verified
Q103: A meal containing a burger, fries, and
Q105: Given the data in the table below,
Q106: A 25.5-g piece of cheddar cheese contains
Q107: Calculate the value of ΔE in joules
Q108: The kinetic energy of a 23.2-g object
Q109: Given the data in the table below,
Q111: Given the data in the table below,
Q113: Given the data in the table below,
Q114: The value of ΔE for a system
Q115: The average fuel value of sugars is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents