Using Bohr's equation for the energy levels of the electron in the hydrogen atom, determine the 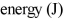 of an electron in the n = 4 level.
of an electron in the n = 4 level.
A) -1.36 × 10-19
B) -5.45 × 10-19
C) -7.34 × 1018
D) -1.84 × 10-29
E) +1.84 × 10-29
Correct Answer:
Verified
Q61: Of the following,_ radiation has the longest
Q79: A mole of yellow photons of wavelength
Q81: When the electron in a hydrogen atom
Q84: The energy (J)required for an electronic transition
Q86: The n = 5 to n =
Q87: The energy (J)required for an electronic transition
Q90: What is the wavelength (angstroms)of a photon
Q91: Calculate the energy (J)change associated with an
Q98: When the electron in a hydrogen atom
Q100: An electron in a Bohr hydrogen atom
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents