Using the table of average bond energies below, the ΔH for the reaction is __________ kJ. 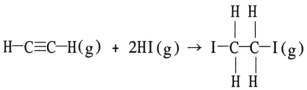 Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
A) +160
B) -160
C) -217
D) -63
E) +63
Correct Answer:
Verified
Q85: How many different types of resonance structures
Q98: Using the table of average bond energies
Q113: The electron configuration that corresponds to the
Q115: The principal quantum number of the electrons
Q116: Using the table of bond dissociation energies,
Q118: How many single covalent bonds must a
Q119: Of the atoms below, _ is the
Q121: The reaction below is used to produce
Q126: Alternative but equivalent Lewis structures are called
Q134: To produce maximum heat, an explosive compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents