Using the table of bond dissociation energies, the ΔH for the following reaction is __________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 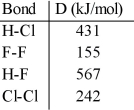
A) -359
B) -223
C) 359
D) 223
E) 208
Correct Answer:
Verified
Q64: In the molecule below,which atom has the
Q69: A nonpolar bond will form between two
Q71: Electronegativity _ from left to right within
Q81: How many equivalent resonance structures can be
Q91: The Lewis structure of PF3 shows that
Q99: In the resonance form of ozone shown
Q102: Of the atoms below, _ is the
Q103: The chloride of which of the following
Q103: Of the bonds below,_ is the least
Q104: Using the table of average bond energies
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents