The Lewis structure of carbon dioxide is given below. The hybridization of the carbon atom in carbon dioxide is __________. 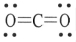
A) sp3
B) sp2
C) sp
D) sp2d
E) sp2d2
Correct Answer:
Verified
Q47: In order to exhibit delocalized π bonding,
Q48: A triatomic molecule cannot be linear if
Q50: Valence bond theory does not address the
Q60: A typical double bond _.
A)is stronger and
Q61: Based on molecular orbital theory, the bond
Q62: Based on molecular orbital theory, the bond
Q71: Based on molecular orbital theory,the only molecule
Q75: Molecular Orbital theory correctly predicts diamagnetism of
Q78: The carbon-carbon σ bond in ethylene, H2C-CH2,
Q84: The hybridization of the oxygen atom labeled
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents