There is/are __________ π bond(s) in the molecule below. 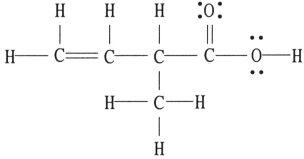
A) 0
B) 1
C) 2
D) 4
E) 16
Correct Answer:
Verified
Q105: The O-S-O bond angle in SO2 is
Q105: There are _ σ and _ π
Q107: There are _ σ and _ π
Q109: The electron-domain geometry of the AsF6- ion
Q114: The hybridization of orbitals on the central
Q120: In order to produce sp2 hybrid orbitals,_
Q132: The molecular geometry of the H3O+ ion
Q134: There is/are _ π bond(s)in the molecule
Q141: Using the VSEPR model, the electron-domain geometry
Q142: According to valence bond theory, which orbitals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents