Of the following, only __________ is impossible for an ideal gas.
A) 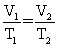
B) V1T1 = V2T2
C) 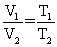
D) 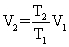
E) 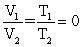
Correct Answer:
Verified
Q1: Which statement about atmospheric pressure is false?
A)As
Q2: The first person to investigate the relationship
Q5: Which of the following equations shows an
Q8: The pressure exerted by a column of
Q11: The pressure exerted by a column of
Q16: One significant difference between gases and liquids
Q18: The volume of an ideal gas is
Q19: Molecular compounds of low molecular weight tend
Q20: Which of the following statements about gases
Q20: If one was told that their blood
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents