The Keq for the equilibrium below is 5.4 × 1013 at 480.0°C. 2NO (g) + O2 (g) 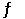 2NO2 (g)
2NO2 (g)
What is the value of Keq at this temperature for the following reaction?
4NO (g) + 2O2 (g) 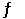 4NO2 (g)
4NO2 (g)
A) 5.4 × 1013
B) 5.4 × 10-13
C) 1.9 × 1012
D) 1.9 × 10-12
E) 2.9 × 1027
Correct Answer:
Verified
Q15: Which of the following expressions is the
Q16: Given the following reaction at equilibrium, if
Q17: The Keq for the equilibrium below is
Q18: The equilibrium constant for reaction 1 is
Q19: The Keq for the equilibrium below is
Q21: Which of the following expressions is the
Q22: The equilibrium constant for the gas phase
Q23: Consider the following equilibrium. 2SO2 (g)+ O2
Q24: Of the following equilibria, only _ will
Q25: Which of the following expressions is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents