The Keq for the equilibrium below is 5.4 × 1013 at 480.0°C. 2NO (g) + O2 (g) 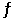 2NO2 (g)
2NO2 (g)
What is the value of Keq at this temperature for the following reaction?
NO2 (g) 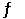 NO (g) + 1/2 O2 (g)
NO (g) + 1/2 O2 (g)
A) 5.4 × 10-13
B) 5.4 × 1013
C) 1.4 × 10-7
D) 5.66 × 10-3
E) none of the above
Correct Answer:
Verified
Q1: In what year was Fritz Haber awarded
Q5: The equilibrium expression for Kp for the
Q9: The Keq for the equilibrium below is
Q11: The equilibrium-constant expression depends on the _
Q13: The value of Keq for the following
Q14: Which one of the following will change
Q14: Which of the following expressions is the
Q15: At equilibrium,_.
A)all chemical reactions have ceased
B)the rates
Q15: Which of the following expressions is the
Q19: Which one of the following is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents