Consider the following reaction at equilibrium: 2SO2 (g) + O2 (g) 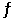 2SO3 (g) ΔH° = -99 kJ
2SO3 (g) ΔH° = -99 kJ
Le Châtelier's principle predicts that an increase in temperature will result in __________.
A) a decrease in the partial pressure of SO3
B) a decrease in the partial pressure of SO2
C) an increase in Keq
D) no changes in equilibrium partial pressures
E) the partial pressure of O2 will decrease
Correct Answer:
Verified
Q23: The effect of a catalyst on an
Q54: Consider the following reaction at equilibrium: 2CO2
Q55: Consider the following chemical reaction: CO (g)+
Q56: The value of Keq for the equilibrium
Q57: Dinitrogentetraoxide partially decomposes according to the following
Q59: Consider the following reaction at equilibrium: C
Q60: The value of Keq for the equilibrium
Q61: Given the following reaction at equilibrium at
Q62: Given the following reaction at equilibrium, if
Q63: Consider the following chemical reaction: H2 (g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents