The equilibrium constant (Kp) for the interconversion of PCl5 and PCl3 is 0.0121: PCl5 (g) 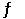 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial pressure of PCl3 is __________ atm.
A) 0.0782
B) 0.0455
C) 0.0908
D) 0.0330
E) 0.123
Correct Answer:
Verified
Q23: The effect of a catalyst on an
Q44: Consider the following reaction at equilibrium: 2NH3
Q45: Consider the following reaction at equilibrium. 2CO2
Q46: The value of Keq for the equilibrium
Q47: The value of Keq for the equilibrium
Q48: The value of Keq for the equilibrium
Q50: Consider the following reaction at equilibrium: 2CO2
Q51: A reaction vessel is charged with hydrogen
Q52: Consider the following reaction at equilibrium: 2NH3
Q54: Consider the following reaction at equilibrium: 2CO2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents