Given the following reaction at equilibrium at 300.0 K: NH4HS (s) 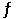 NH3 (g) + H2S (g)
NH3 (g) + H2S (g)
If pNH3 = pH2S = 0.109 atm, Kp = __________.
A) .0119
B) 4.99 × 10-4
C) .109
D) .0821
E) 5.66 × 10-3
Correct Answer:
Verified
Q56: The value of Keq for the equilibrium
Q57: Dinitrogentetraoxide partially decomposes according to the following
Q58: Consider the following reaction at equilibrium: 2SO2
Q59: Consider the following reaction at equilibrium: C
Q60: The value of Keq for the equilibrium
Q62: Given the following reaction at equilibrium, if
Q63: Consider the following chemical reaction: H2 (g)+
Q64: At elevated temperatures, molecular hydrogen and molecular
Q65: At 27°C, Kp = 0.095 for the
Q66: At 1000.0 K, the equilibrium constant for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents