Nitrosyl bromide decomposes according to the following equation. 2NOBr (g) 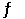 2NO (g) + Br2 (g)
2NO (g) + Br2 (g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or Br2. At equilibrium the flask contained 0.36 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
A) .28,.28
B) .36,.18
C) .28,.14
D) .14,.23
E) .36,.36
Correct Answer:
Verified
Q63: Consider the following chemical reaction: H2 (g)+
Q64: At elevated temperatures, molecular hydrogen and molecular
Q65: At 27°C, Kp = 0.095 for the
Q66: At 1000.0 K, the equilibrium constant for
Q67: Given the following reaction at equilibrium at
Q69: Kp = 0.0198 at 721 K for
Q71: In the coal-gasification process, carbon monoxide is
Q72: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q73: At 200°C, the equilibrium constant (Kp)for the
Q82: The equilibrium-constant expression for a reaction written
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents