The first law of thermodynamics can be given as __________.
A) ΔE = q + w
B) 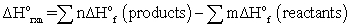
C) for any spontaneous process, the entropy of the universe increases
D) the entropy of a pure crystalline substance at absolute zero is zero
E) ΔS = qrev/T at constant temperature
Correct Answer:
Verified
Q1: ΔS is positive for the reaction _.
A)2NO
Q3: The thermodynamic quantity that expresses the degree
Q7: Which one of the following correctly indicates
Q10: A reversible process is one that _.
A)can
Q10: ΔS is positive for the reaction _.
A)2H2
Q12: Which one of the following processes produces
Q14: Consider a pure crystalline solid that is
Q16: Which of the following statements is true?
A)Processes
Q17: Which one of the following is always
Q18: The entropy of the universe is _.
A)constant
B)continually
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents