The second law of thermodynamics states that __________.
A) ΔE = q + w
B) 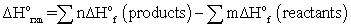
C) for any spontaneous process, the entropy of the universe increases
D) the entropy of a pure crystalline substance is zero at absolute zero
E) ΔS = qrev/T at constant temperature
Correct Answer:
Verified
Q1: The entropy change accompanying any process is
Q7: ΔS is positive for the reaction _.
A)CaO
Q9: When a system is at equilibrium,_.
A)the reverse
Q13: A reaction that is spontaneous as written
Q15: For an isothermal process, ΔS = _.
A)q
B)qrev/T
C)qrev
D)Tqrev
E)q
Q19: Which of the following statements is false?
A)The
Q21: For a reaction to be spontaneous under
Q23: Of the following, the entropy of _
Q24: Given the following table of thermodynamic data,
Q34: ΔS is negative for the reaction _.
A)2SO2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents