The equilibrium constant for the following reaction is 3.5 × 108 at 25°C. N2 (g) + 3H2 (g) 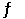 2NH3 (g)
2NH3 (g)
The value of ΔG° for this reaction is __________ kJ/mol.
A) 22
B) -4.1
C) 4.1
D) -49
E) -22
Correct Answer:
Verified
Q64: The value of ΔG° at 25 °C
Q72: The value of ΔG° at 25 °C
Q99: The standard Gibbs free energy of formation
Q103: A reversible change produces the maximum amount
Q104: The equilibrium constant for a reaction is
Q105: Phosphorous and chlorine gases combine to produce
Q112: Calculate ΔG° for the autoionization of water
Q123: The more negative ΔG° is for a
Q124: The vaporization of a substance at its
Q125: The quantity of energy gained by a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents