The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by __________.
A) ΔG = 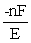
B) ΔG = 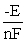
C) ΔG = -nFE
D) ΔG = -nRTF
E) ΔG = 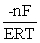
Correct Answer:
Verified
Q56: The standard cell potential (E°cell)of the reaction
Q76: The standard emf for the cell using
Q77: The standard cell potential (E°cell)for the voltaic
Q78: How many kilowatt-hours of electricity are used
Q79: The standard cell potential (E°)of a voltaic
Q81: How many minutes will it take to
Q82: The electrolysis of molten AlCl3 for 3.25
Q84: In the formula ΔG = -nFE, F
Q85: How many minutes will it take to
Q105: When iron is coated with a thin
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents