Carbon-11 decays by positron emission: 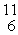 C →
C → 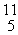 B +
B + 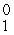 e The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes this radioactive decay, __________ g of mass is converted to energy.
e The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes this radioactive decay, __________ g of mass is converted to energy.
A) 1.16 × 10-3
B) 3.48 × 105
C) 1.16 × 10-6
D) 8.62 × 102
E) 1.28 × 10-2
Correct Answer:
Verified
Q124: 
Q127: How much energy (in J)is produced when
Q142: Conversion of one nucleus into another was
Q145: What happens to the atomic mass number
Q146: The first nuclear transmutation resulted in the
Q147: What happens in the nucleus of an
Q148: What is the predominant isotope of uranium?
Q151: What is the source of the tremendous
Q152: Carbon-11,fluorine-18,oxygen-15 and nitrogen-13 are all used in
Q159: What was the purpose of the Manhattan
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents