Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 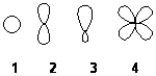
A) 1
B) 2
C) 3
D) 4
Correct Answer:
Verified
Q43: How many electrons are there in the
Q51: What is the approximate value of
Q52: Which of the following statements is not
Q53: Which of the following statements is not
Q54: Rank the following in order of decreasing
Q56: What is the approximate value of
Q57: Which of the following resonance structures is
Q58: Which atomic orbitals overlap to form the
Q60: Which of the following statements is not
Q60: Which atomic orbitals overlap to form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents