Consider the structure of urea given below. 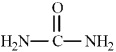 To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
Correct Answer:
Verified
Q81: The maximum number of electrons that a
Q84: The approximate H-C-H bond angle in methane
Q85: The following molecules all contain the same
Q87: The formal charge on carbon in carbon
Q89: Draw bond-line structures of all of the
Q91: Provide a neatly drawn figure to show
Q92: The formal charges in the complex should
Q97: The most electronegative elements in the periodic
Q99: The following species forms during an organic
Q100: In drawing the Lewis structure for an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents