The following is generic depiction of a reaction using the curve arrow formalism. 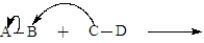 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
Correct Answer:
Verified
Q65: A reaction in which the free energy
Q66: Use curved arrows to show the movement
Q67: Complete the equation below for the protonation
Q68: Which acid has the strongest conjugate base?
Q69: Consider the following reaction coordinate diagram.
Q71: A reaction where
Q72: Which acid will be almost completely deprotonated
Q73: Consider the following reaction coordinate diagram.
Q74: Use curved arrows to show the movement
Q75: Which acid has the smallest Ka?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents