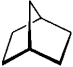
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:
Verified
Q42: What is the hydrogen deficiency index for
Q55: What is the hydrogen deficiency index for
Q56: Which of the following compounds gives a
Q56: Which of the carbon atoms in the
Q57: Which of the following compounds have enantiotopic
Q62: For each of the compounds below tell
Q63: Which C8H10 compound gives the following 1H
Q64: The following compound will have a 1H
Q65: Consider the compound shown below. 
Q66: Which C8H10 compound gives the following 1H
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents