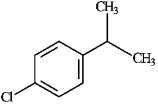
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:
Verified
Q66: Nuclear magnetic resonance spectroscopy provides information about
Q70: Which C9H10O compound gives the following 1H
Q71: Which C6H12O2 compound gives the following 1H
Q72: Which C6H12O2 compound gives the following 1H
Q73: Predict the splitting of each of the
Q76: For each of the compounds below tell
Q77: For each of the compounds below tell
Q78: Consider the following structure. Q79: Which C9H10O compound gives the following 1H Q80: The splitting of signals in the 1H![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents