Using the symbol δ+and δ-, show the direction of the polarity in the indicated bond. 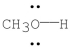
Correct Answer:
Verified
Q1: Give the electronic configuration for Ca+2.
Q3: Which of the compounds below have bonds
Q4: What type of bonding is most important
Q5: Which of the following elements does this
Q11: The formal charge on nitrogen in the
Q12: Provide the mathematical equation for the dipole
Q13: Which of the following is the electronic
Q16: Ar,K+,Cl- are isoelectronic elements (elements with the
Q17: Which of the following statements correctly describes
Q20: Give the electronic configuration for N-3.
A)1s22s2
B)1s22s22p3
C)1s22s22p4
D)1s22s22p6
E)1s22s22p63s1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents