What kind of molecular orbital (σ, σ*, π, or π*)results when the two atomic orbitals shown below interact in the manner indicated? 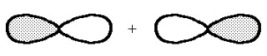
Correct Answer:
Verified
Q5: Consider the interaction of two hydrogen 1s
Q22: Both sigma (σ)and pi (π)bonds can be
Q27: The Kekulé structure of pentane is shown
Q29: What are the formal charges on nitrogen
Q29: Expand the condensed structure below to show
Q30: Which of the following is the most
Q32: Which of the following structures, including formal
Q33: What kind of molecular orbital (σ, σ*,
Q36: Draw the Kekulé structure for each of
Q36: What kind of molecular orbital (σ, σ*,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents