Which of the following is an sp2 hybridized carbon?
A) 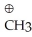
B) ∙ CH3
C) 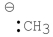
D) A and B
E) A, B and C
Correct Answer:
Verified
Q30: Draw the Lewis structure for CH3N2+.
Q38: Give the formal charge on nitrogen in
Q40: Draw a Lewis structure for the molecule
Q45: Which carbon(s)in the following molecule is (are)sp
Q47: Among the hydrogen halides,the strongest bond is
Q48: The lone-pair electrons of the methyl anion
Q50: The N-H bond in the ammonium ion,NH4+,is
Q56: How many sp2 hybridized carbons are present
Q57: What orbitals overlap to create the C-H
Q79: Draw condensed structures for the four compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents