Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
A) 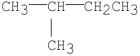
B) CH3CH2CH2CH3
C) 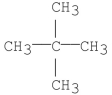
D) CH3CH2CH2CH2CH3
E) 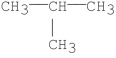
Correct Answer:
Verified
Q52: Consider the three isomeric alkanes n-hexane, 2,3-dimethylbutane,
Q62: Assuming roughly equivalent molecular weights,which of the
Q64: Would you expect sodium chloride (NaCl)to be
Q67: Which of the following is the staggered
Q68: Which of the following is the most
Q70: What is polarizability and how is it
Q73: Which of the following would have the
Q76: What is the strongest intermolecular force present
Q105: Which compound is more soluble in water?
Q117: Which compound is more soluble in water?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents