The compound below contains an asymmetric center at nitrogen. Why can't individual stereoisomers of this compound be isolated at room temperature? 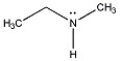
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q102: Label each asymmetrical carbon in the compound
Q105: Assign an R or S configurational label
Q106: Identify the pair of compounds. 
Q108: Identify the compounds that have an asymmetric
Q108: Draw any diastereomer of (2R,3R)-2,3-dichloropentane.Be careful to
Q109: In the Fischer projection below, what are
Q111: Give the complete name(s)of the following compound.
Q114: Draw a perspective formula of (2R,3S)-3-bromo-2-butanol.
Q118: Can one predict whether a compound with
Q127: Briefly describe how two enantiomers might be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents